News
What’s new in the world of PWS, the disability and education sectors? Plus, PWSA news, details of new resources and advocacy updates.
NEWSLETTERS
November 2024
September 2024
July 2024
May 2024 bulletin
April 2024
February 2024
December 2023
October 2023
July 2023
April 2023 bulletin
March 2023
February 2023
December 2022
November 2022
October 2022 bulletin
September 2022
July 2022 bulletin
June 2022
March 2022
February 2022
December 2021
November 2021
September 2021
July 2021
May 2021
February 2021
December 2020
November 2020
July 2020
May 2020
March 2020
December 2019
November 2019
August 2019
June 2019
February 2019
December 2018
November 2018
December 2017
October 2017
July 2017
May 2017
April 2017
February 2017
CATEGORIES
NEWS POSTS
PWSA Family Support Camp – Taupō, March 2025
In March this year, we returned to MiCamp Taupō where we were treated to lakeside sunshine once again. This was our largest camp to date with 94 people registering to…
PWSA(NZ) Transition Symposium
We held our first transition event during October, with one day focused on support agencies and providers, and the other day focused on families. We hope the event was beneficial…
6th Asia Pacific PWS Conference – Sydney
APPWS2024 was jointly hosted by PWS Australia and PWRFA (Prader-Willi Research Foundation Australia), August 30th - 31st, 2024. A number of delegates attended from New Zealand, who were mostly speaking,…
PWSA Camp, Taupō – March 2023
Our Family Camp finally took place after a 3 year gap due to Covid disruptions. It was lovely to return to MiCamp Taupō where the sunshine came out for us…
Young Families Weekend 2022
On the weekend of September 17th and 18th, our Young Families Weekend finally took place after unfortunate Covid-19 postponements - it was fantastic to be able to connect with others…
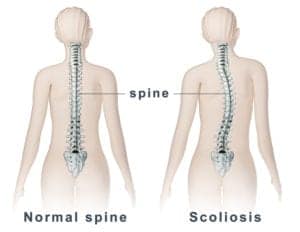
Scoliosis Care in New Zealand
We are aware that some children with scoliosis are not being diagnosed until their curve is quite severe. This is concerning because an earlier diagnosis can lead to treatments that…
PWSA(NZ) stands with Rare Disorders NZ in demanding Fair for Rare
On International Rare Disease Day, PWSA(NZ) stands with Rare Disorders NZ calling for action from Government to commit to a National Health Strategy for Rare Disorders. Today, Rare Disorders NZ…
5th Asia Pacific PWS Conference
Together with the PWS Associations of Australia, Malaysia and Thailand, we held our first virtual conference in October 2021, which was also the first Asia Pacific conference to be co-organised…
Transformative Change to Disability System Announced
The Government has announced long awaited news regarding planned changes as part of disability system transformation. The key changes announced were: • The establishment of a Ministry for Disabled People…
New Resource – Guides for Doctors: Consensus Documents
IPWSO's Clinical and Scientific Advisory Board have produced some excellent new guides for doctors and health practitioners to aid the medical evaluation of patients. They provide an overview of the…
Tweens and Teens Weekend 2021
At the end of May, we enjoyed a weekend in Wellington attended by 12 of our tweens and teens with an accompanying parent. Many of these teens know each other…
Leadership and Social Weekend for Adults Living with PWS 2020
We invited our adult members with PWS to a Leadership and Social Weekend which was held Oct 16th - 18th 2020, at The Surrey Hotel in Auckland. The programme was…
PWSA Family Camp, Taupo – Feb/March 2020
Just a couple of weeks before our world changed momentously with the impact of Covid-19, we were able to enjoy a sunny weekend together on the alluring shores of Lake…
Julia and Dane interview Dr Jennifer Miller at IPWSO 2019 Conference
Whilst attending the IPWSO conference in Cuba, Julia and Dane Fuller, from New Zealand, were very fortunate to be able to interview expert endocrinologist, Dr Jennifer Miller. The interview contains…
Young Families Weekend – Nov 2019
We have recently held Young Families Weekends approximately every 2 years. Our latest weekend for families of newly diagnosed or young children was held at the Top 10 Holiday Park…
Teens Weekend – May 2019
Following the success of our previous Tweens and Teens Weekend in 2017, we recently held another weekend, this time in Wellington. Our weekend programme was full of activities which included…
Self-funding GH treatment for adults now costs much less…
There is evidence that for people with PWS it is beneficial to continue treatment with growth hormone therapy even after they have reached their final height. To learn more about…
PWSA Family Camp – May 2018
Thank you to all the families and carers who joined us at YMCA Camp Adair, Hunua, Auckland. We think it was our biggest camp yet with 93 people booked to…
Join the Conversation – Let’s Talk about Education!
Have your say.... The Minister of Education has announced a three-year review of the public education system. Plans include a review of NCEA, a review of Tomorrow’s Schools, developing a…
May is PWS Awareness Month!
May is international PWS Awareness Month. Each year we focus on spreading awareness during May with the belief that awareness leads to increased understanding and acceptance. There are lots of…
ADVOCACY UPDATES
Nov 2025 – Pharmac proposal to decline medicines on OFI
PHARMAC are proposing to decline medicines that have been in the lowest-ranked group for more than two years. This would apply to the bottom 20% of applications (if there are more than 100), or the bottom 10% (if fewer than 100).
PWSA strongly opposes this proposal and would like greater transparency about medicine funding decisions instead.
PWSA(NZ) Submission to Pharmac Nov 2025
Sept 2025 – Disability Strategy 2026-2030 Consultation
The New Zealand Disability Strategy 2016-2026 is due to be refreshed and we have submitted feedback to the public consultation on the draft strategy proposed for 2026-2030. The draft sets out a vision for the future, contains 5 priority outcome areas with actions in education, employment, health, housing and justice, and describes how success will be measured.
PWSA(NZ) Disability Strategy Submission to Whaikaha
March 2025 – DSS Consultation on Disability Supports
We participated in the DSS community consultation on assessment tools and processes, and on options for flexible funding. The Government will now make decisions on stabilising support services before considering work to strengthen supports into the future. We expect to hear more later this year.
PWSA(NZ) Submission to DSS Consultation – March 2025
March 2025 – Proposal to DSS: PWS specific residential care
In March we met with the DSS taskforce to discuss our proposal for more PWS specific residential care options. The taskforce accepted our proposal document, which is now being reviewed by the DSS commissioning team.
PWSA(NZ) Proposal for PWS specific residential care
Feb 2024 – Request for Review of Scoliosis Screening
Following concerns about late diagnosis of scoliosis in some patients with PWS, we sought the advice of international expert Dr Harold van Bosse, and wrote to New Zealand’s orthopaedic, endocrine and paediatric groups to request a review of screening practice and suggesting the implementation of a radiographic screening programme for patients with PWS in New Zealand similar to that described by Dr van Bosse.
PWSA(NZ) letter re. scoliosis screening
Nov 2023 – Pharmac proposal to fund testosterone gel
We made a submission on Pharmac’s proposal to widen testosterone treatment types to include a gel option. Since the discontinuation of Andriol, a new capsule brand is not available to those first prescribed testosterone after Nov 2021 at this stage. Our submission requested that a tablet / capsule form of testosterone be made available to all patients with PWS.
PWSA(NZ) Submission to PHARMAC – Testosterone
Feb 2024 PHARMAC Response: “Unfortunately, we were unable to secure a Medsafe approved testosterone capsule product from this procurement process. We will continue to explore options that could address an unmet health need for people requiring testosterone.”
March 2023 – Adult Decision-Making Capacity Law
Te Aka Matua o te Ture | the New Zealand Law Commision are reviewing how the law should respond when capacity for decision-making is affected and seek feedback on how the law could be improved. The PWSA submitted a response outlining some concerns specific to Prader-Willi Syndrome. The Law Commission are planning to publish a second issues paper in the second half of 2023. This will contain their detailed analysis of issues with the current law, and they will seek feedback on options for reform.
PWSA Submission to the Law Commission
Update June 2024 – Our response to the Second Issues Paper mainly focuses on Q.6 and the the proposed single test for decision-making capacity which identifies four factors for assessment: PWSA Submission on Second Issues Paper
March 2022 – Education Action – Highest Needs Review
The Highest Needs Review is taking place as part of a commitment outlined in Priority 4 in the Learning Support Action Plan 2019-2025: flexible supports for neurodiverse children and young people. The review team will develop a report for the Minister with recommendations for Cabinet in October 2022.
PWSA(NZ) Submission to the Highest Needs Review
March 2022 – Covid-19 Antivirals Consultation
PHARMAC announced a brief consultation period on the eligibility criteria for 2 oral antiviral medications due to arrive in New Zealand between the end of April and June: Pfizer’s Paxlovid and Merck’s Molnupiravir. The supply will be limited and the restrictive criteria proposed would exclude some of our most vulnerable members. Two days after feedback closed, PHARMAC announced tightening of the criteria for Remdesivir, an antiviral administered via injection. As we wait to hear confirmation of the criteria for the oral antivirals, we wait in hope that access criteria will not follow suit in being made more restrictive.
PWSA(NZ) Submission to PHARMAC – Covid-19 antivirals
Dec 2021 – Pae Ora Bill – Health System Reform
A planned health reform will disestablish the DHBs in place of a new structure with new accountability arrangements. The Pae Ora Bill outlines the core changes planned, which include the establishment of Health New Zealand, the Māori Health Authority, and specific health strategies for hauora Māori, Pacific health, and the health of disabled people. The PWSA(NZ) made a submission on this Bill seeking acknowledgement that the rare disorder population are a significant population group that have been overlooked in this Bill. We requested that a health strategy for rare disorders be added in order for the Bill to align with its purpose of improving the health of all New Zealanders and achieving equity. We also did not support clauses which exclude Pharmac from the principles in this Bill surrounding equity and engagement with population groups. We asked that equitable performance be expected from Pharmac and that legislation be amended to reflect issues raised in the recently published Pharmac Review Interim Report.
PWSA(NZ) Submission to Parliament on the Pae Ora Bill
Oct 2021 – Content of 2023 of Disability Survey
Stats NZ consulted on the content of the 2023 Disability Survey, the first since 2013. This survey is the main source of information for estimating the number of disabled people in New Zealand and provides information on how well they are faring across a range of outcomes. Government and other organisations use this data to understand the needs of disabled people and plan services for them. Therefore, it is important to provide feedback on the content of the survey to ensure relevant data is collected from the PWS and rare disorder communities.
PWSA(NZ) Submission to Stats NZ
July 2021 – Medicines Access – Review of PHARMAC
The Government followed through on an election promise to conduct an independent review into PHARMAC (Pharmaceutical Management Agency). Feedback was sought to contribute to this review process and help form a proposal on how PHARMAC can best serve the needs of New Zealanders. The review is due in December.
PWSA(NZ) Submission to the PHARMAC Review Panel
Pharmac Review Interim Report – published Dec 2021
July 2017 – Growth Hormone Access
Our submission to Pharmac to widen access until 18 years has been declined. We anticipate new evidence becoming available and we intend to keep trying. In the meantime, please contact us: a.) if your child has stopped GHT and you have observed effects on energy level, body composition, weight gain, mood or overall quality of life; b.) if your child later resumed treatment and you observed benefits; or c.) if your child started treatment as a teenager or adult.
Submission to PTAC – February 2017
Dec 2016 – Education Action – Learning Support Update
On November 24, Hekia Parata announced the next steps in the Learning Support Update with the proposed changes to be phased in with a pilot beginning in the Bay of Plenty early 2017. We offered input to the changes in the form of letters to Parliament, writing to all MPs with education or disability portfolios and Education and Science Select Committee members. We also sent letters to other political party MPs seeking comment.
PWSA(NZ) Letter to MPs
Dec 2016 – Growth Hormone Access
Pharmac considered submissions and removed the growth velocity barrier and allowed children to start treatment from 6 months old without the need for collecting growth data – in effect from Jan 2017. Unfortunately, extending treatment until 18 years was not approved. We met with Pharmac in September to discuss our case with senior staff, including Medical Director Dr John Wyeth. We were advised that to achieve our goal of funded GH for all people with PWS, a step by step approach is recommended - under 2’s, up to 18 years, and then adults. We are following this advice. Thank you to all who made submissions.
PWSA(NZ) Submission to PHARMAC – Sept 2016
Nov 2016 – Education Action – Education Amendment Bill
We submitted a response to the Education (Update) Amendment Bill and were given an opportunity to speak to our submission before the Select Committee, December 2016. We also forwarded questions to Kelly Dugan of SmileDial for his meeting with the Minister for Disability Issues and MoE staff members.
PWSA(NZ) Submission – Education Amendment Bill
Sept 2016 – Education Action – YouthLaw Research
The PWSA helped to fund research by YouthLaw into access to education for children with special educational needs. Their report was published in Sept 2016 and is an excellent summary of the problems facing our children.
YouthLaw Report: Challenging the Barriers
Sept 2016 – Growth Hormone Access
Pharmac advised their Endocrinology Subcommittee recommended access for funded GHT is widened to include children with PWS from 6 months old and the requirement for slow growth be removed, but felt there was insufficient evidence to extend treatment to 18 years. The recommendation went to PTAC in November to endorse or decline. When approved by PTAC, it required funding approval and we made another submission regarding funding priority. Having voiced concerns regarding timeframes and our disappointment regarding some decisions, Pharmac agreed to a meeting. We also met with NZORD rare disease advocates to see how they can help.
PWSA(NZ) Submission to PHARMAC – Dec 2015
Dec 2015 – Education Action – Education for All
In November 2015 we consulted with members on key educational issues affecting our children with PWS. We took those concerns to a meeting with IHC advocates, Education for All and other disability support groups. Following this first meeting, issues raised and possible solutions discussed were to be taken to further meetings with main bodies in the education sector and then to the MoE. We also made a submission to the update of the 1989 Education Act.
PWSA(NZ) Submission – Education Act Update
Sept 2015 – Education Action – Select Committee Inquiry
We submitted a response to the Education and Science Select Committee’s Inquiry into the Identification and Support for Students with the Significant Challenges of Dyslexia, Dyspraxia and Autism Spectrum Disorders. The inquiry report was vaguely promising, but many areas of concern remained.
PWSA(NZ) Submission to the Select Committee Inquiry